What Tool Measures Air Pressure

Example of the widely used Bourdon pressure level gauge

Checking tire pressure with a bound and piston tire-pressure level judge
Pressure measurement is the measurement of an applied force by a fluid (liquid or gas) on a surface. Pressure is typically measured in units of force per unit of area. Many techniques have been developed for the measurement of pressure and vacuum. Instruments used to measure and display pressure level mechanically are called pressure gauges, vacuum gauges or compound gauges (vacuum & pressure). The widely used Bourdon judge is a mechanical device, which both measures and indicates and is probably the best known type of gauge.
A vacuum guess is used to measure pressures lower than the ambient atmospheric pressure, which is set as the null signal, in negative values (for case, −1 bar or −760 mmHg equals total vacuum). Virtually gauges mensurate pressure relative to atmospheric pressure as the null signal, so this form of reading is simply referred to as "gauge pressure". However, anything greater than total vacuum is technically a form of pressure. For very low pressures, a gauge that uses full vacuum as the naught point reference must be used, giving force per unit area reading as an absolute pressure.
Other methods of pressure measurement involve sensors that tin transmit the force per unit area reading to a remote indicator or control system (telemetry).
Absolute, gauge and differential pressures — zero reference [edit]

Natural gas pressure level judge
Everyday pressure measurements, such equally for vehicle tire pressure, are normally made relative to ambience air force per unit area. In other cases measurements are made relative to a vacuum or to another specific reference. When distinguishing between these zero references, the following terms are used:
- Absolute force per unit area is nix-referenced against a perfect vacuum, using an absolute scale, and so it is equal to approximate pressure plus atmospheric force per unit area.
- Approximate force per unit area is goose egg-referenced against ambient air force per unit area, then it is equal to absolute pressure minus atmospheric pressure level.
- Differential pressure is the difference in pressure between 2 points.
The nothing reference in employ is usually implied by context, and these words are added only when description is needed. Tire pressure and blood pressure are gauge pressures by convention, while atmospheric pressures, deep vacuum pressures, and altimeter pressures must exist absolute.
For nigh working fluids where a fluid exists in a closed organisation, approximate pressure measurement prevails. Pressure instruments connected to the system will indicate pressures relative to the electric current atmospheric pressure. The state of affairs changes when extreme vacuum pressures are measured, then absolute pressures are typically used instead and measuring instruments used will be unlike.
Differential pressures are commonly used in industrial process systems. Differential pressure gauges have two inlet ports, each connected to one of the volumes whose pressure is to exist monitored. In result, such a judge performs the mathematical operation of subtraction through mechanical means, obviating the demand for an operator or control arrangement to lookout two dissever gauges and determine the difference in readings.
Moderate vacuum force per unit area readings can exist ambiguous without the proper context, as they may correspond absolute force per unit area or estimate pressure level without a negative sign. Thus a vacuum of 26 inHg gauge is equivalent to an accented pressure of 4 inHg, calculated as thirty inHg (typical atmospheric pressure) − 26 inHg (gauge pressure).
Atmospheric pressure is typically about 100 kPa at sea level, only is variable with altitude and weather. If the absolute pressure of a fluid stays constant, the gauge force per unit area of the same fluid will vary equally atmospheric pressure changes. For instance, when a car drives up a mountain, the (gauge) tire force per unit area goes upwardly because atmospheric force per unit area goes downwards. The absolute pressure in the tire is essentially unchanged.
Using atmospheric pressure every bit reference is usually signified by a "grand" for judge after the pressure unit of measurement, east.g. 70 psig, which means that the pressure measured is the total pressure minus atmospheric pressure. There are two types of gauge reference pressure: vented guess (vg) and sealed gauge (sg).
A vented-gauge force per unit area transmitter, for example, allows the outside air pressure level to be exposed to the negative side of the pressure-sensing diaphragm, through a vented cable or a hole on the side of the device, so that it always measures the pressure referred to ambience barometric pressure. Thus a vented-gauge reference pressure sensor should always read zippo pressure when the process pressure connexion is held open to the air.
A sealed gauge reference is very similar, except that atmospheric pressure is sealed on the negative side of the diaphragm. This is normally adopted on high pressure ranges, such as hydraulics, where atmospheric pressure changes volition have a negligible effect on the accuracy of the reading, so venting is not necessary. This too allows some manufacturers to provide secondary pressure level containment equally an extra precaution for pressure equipment prophylactic if the burst pressure of the principal force per unit area sensing diaphragm is exceeded.
There is another way of creating a sealed guess reference, and this is to seal a high vacuum on the opposite side of the sensing diaphragm. So the output betoken is offset, so the pressure sensor reads close to goose egg when measuring atmospheric pressure.
A sealed gauge reference pressure transducer will never read exactly zero considering atmospheric pressure is always changing and the reference in this case is fixed at ane bar.
To produce an absolute pressure sensor, the manufacturer seals a high vacuum behind the sensing diaphragm. If the procedure-force per unit area connection of an accented-pressure transmitter is open to the air, it will read the actual barometric pressure.
History [edit]
For much of man history, the pressure of gases like air was ignored, denied, or taken for granted, but as early as the 6th century BC, Greek philosopher Anaximenes of Miletus claimed that all things are made of air that is simply changed by varying levels of pressure. He could observe water evaporating, changing to a gas, and felt that this applied even to solid affair. More condensed air fabricated colder, heavier objects, and expanded air made lighter, hotter objects. This was akin to how gases actually practice get less dumbo when warmer, more dumbo when cooler.
In the 17th century, Evangelista Torricelli conducted experiments with mercury that allowed him to measure the presence of air. He would dip a glass tube, closed at one end, into a bowl of mercury and raise the closed end up out of it, keeping the open cease submerged. The weight of the mercury would pull it down, leaving a fractional vacuum at the far terminate. This validated his belief that air/gas has mass, creating pressure level on things around it. Previously, the more pop determination, fifty-fifty for Galileo, was that air was weightless and it is vacuum that provided force, as in a siphon. The discovery helped bring Torricelli to the conclusion:
We alive submerged at the lesser of an sea of the chemical element air, which by unquestioned experiments is known to take weight.
This examination, known equally Torricelli's experiment, was essentially the first documented pressure level gauge.
Blaise Pascal went farther, having his blood brother-in-law try the experiment at unlike altitudes on a mountain, and finding indeed that the farther downwardly in the body of water of temper, the higher the pressure.
Units [edit]
| Pascal | Bar | Technical atmosphere | Standard atmosphere | Torr | Pound per square inch | |
|---|---|---|---|---|---|---|
| (Pa) | (bar) | (at) | (atm) | (Torr) | (lbf/intwo) | |
| 1 Pa | ane Pa ≡ 1 Pa | 1 Pa = ten−5 bar | ane Pa = one.0197×x−5 at | 1 Pa = 9.8692×10−vi atm | 1 Pa = 7.5006×10−3 Torr | ane Pa = 0.000 145 037 737 730 lbf/inii |
| 1 bar | 10five | ≡ 100 kPa ≡ 106 dyn/cmii | = i.0197 | = 0.98692 | = 750.06 | = 14.503 773 773 022 |
| 1 at | 98066.v | 0.980665 | ≡ 1 kgf/cm2 | 0.967 841 105 354 1 | 735.559 240 1 | 14.223 343 307 120 three |
| 1 atm | ≡ 101325 | ≡ i.01325 | 1.0332 | 1 | 760 | fourteen.695 948 775 514 2 |
| i Torr | 133.322 368 421 | 0.001 333 224 | 0.001 359 51 | i / 760 ≈ 0.001 315 789 | i Torr ≈ 1 mmHg | 0.019 336 775 |
| 1 lbf/in2 | 6894.757 293 168 | 0.068 947 573 | 0.070 306 958 | 0.068 045 964 | 51.714 932 572 | ≡ 1 lbf/in2 |

The SI unit of measurement for pressure is the pascal (Pa), equal to i newton per square metre (N·thousand−2 or kg·m−1·s−2). This special name for the unit was added in 1971; before that, pressure in SI was expressed in units such as N·m−ii. When indicated, the nothing reference is stated in parenthesis following the unit, for case 101 kPa (abs). The pound per foursquare inch (psi) is still in widespread use in the US and Canada, for measuring, for instance, tire pressure. A letter is often appended to the psi unit to indicate the measurement's zero reference; psia for absolute, psig for judge, psid for differential, although this practise is discouraged past the NIST.[1]
Because pressure was one time normally measured by its ability to readapt a column of liquid in a manometer, pressures are oftentimes expressed as a depth of a particular fluid (e.thousand., inches of water). Manometric measurement is the field of study of force per unit area head calculations. The most common choices for a manometer's fluid are mercury (Hg) and h2o; h2o is nontoxic and readily available, while mercury's density allows for a shorter cavalcade (and so a smaller manometer) to mensurate a given pressure. The abbreviation "W.C." or the words "water column" are often printed on gauges and measurements that apply water for the manometer.
Fluid density and local gravity can vary from one reading to another depending on local factors, and so the height of a fluid cavalcade does not ascertain force per unit area precisely. Then measurements in "millimetres of mercury" or "inches of mercury" can be converted to SI units as long as attention is paid to the local factors of fluid density and gravity. Temperature fluctuations change the value of fluid density, while location can affect gravity.
Although no longer preferred, these manometric units are still encountered in many fields. Blood force per unit area is measured in millimetres of mercury (run across torr) in nearly of the earth, fundamental venous pressure and lung pressures in centimeters of water are withal common, as in settings for CPAP machines. Natural gas pipeline pressures are measured in inches of h2o, expressed as "inches Due west.C."
Underwater defined employ manometric units: the ambient pressure is measured in units of metres sea water (msw) which is defined as equal to one tenth of a bar. [two] [3] The unit used in the US is the foot sea water (fsw), based on standard gravity and a sea-water density of 64 lb/ftiii. According to the US Navy Diving Manual, one fsw equals 0.30643 msw, 0.030643 bar, or 0.44444 psi,[ii] [3] though elsewhere information technology states that 33 fsw is 14.vii psi (1 atmosphere), which gives ane fsw equal to about 0.445 psi.[iv] The msw and fsw are the conventional units for measurement of diver pressure level exposure used in decompression tables and the unit of measurement of scale for pneumofathometers and hyperbaric bedroom force per unit area gauges.[5] Both msw and fsw are measured relative to normal atmospheric pressure.
In vacuum systems, the units torr (millimeter of mercury), micron (micrometer of mercury),[6] and inch of mercury (inHg) are well-nigh unremarkably used. Torr and micron commonly indicates an absolute pressure, while inHg normally indicates a judge pressure.
Atmospheric pressures are normally stated using hectopascal (hPa), kilopascal (kPa), millibar (mbar) or atmospheres (atm). In American and Canadian engineering, stress is oft measured in kip. Note that stress is non a truthful pressure level since it is not scalar. In the cgs system the unit of pressure was the barye (ba), equal to 1 dyn·cm−2. In the mts arrangement, the unit of measurement of pressure was the pieze, equal to i sthene per foursquare metre.
Many other hybrid units are used such as mmHg/cmii or grams-force/cm2 (sometimes as [[kg/cm2]] without properly identifying the strength units). Using the names kilogram, gram, kilogram-force, or gram-strength (or their symbols) as a unit of force is prohibited in SI; the unit of forcefulness in SI is the newton (N).
Static and dynamic force per unit area [edit]
Static pressure is uniform in all directions, so pressure measurements are independent of direction in an immovable (static) fluid. Period, however, applies additional force per unit area on surfaces perpendicular to the menstruum management, while having little touch on surfaces parallel to the period direction. This directional component of pressure in a moving (dynamic) fluid is chosen dynamic pressure. An musical instrument facing the flow direction measures the sum of the static and dynamic pressures; this measurement is chosen the full pressure or stagnation pressure. Since dynamic pressure is referenced to static force per unit area, information technology is neither estimate nor accented; it is a differential pressure.
While static guess pressure is of main importance to determining net loads on pipage walls, dynamic pressure is used to mensurate flow rates and airspeed. Dynamic force per unit area can exist measured by taking the differential pressure between instruments parallel and perpendicular to the flow. Pitot-static tubes, for instance perform this measurement on airplanes to determine airspeed. The presence of the measuring musical instrument inevitably acts to divert flow and create turbulence, so its shape is critical to accuracy and the calibration curves are ofttimes non-linear.
Applications [edit]
- Altimeter
- Barometer
- Depth gauge
- MAP sensor
- Pitot tube
- Sphygmomanometer
Instruments [edit]

Many instruments have been invented to measure pressure, with different advantages and disadvantages. Pressure range, sensitivity, dynamic response and cost all vary by several orders of magnitude from one instrument blueprint to the side by side. The oldest type is the liquid column (a vertical tube filled with mercury) manometer invented past Evangelista Torricelli in 1643. The U-Tube was invented by Christiaan Huygens in 1661.
Hydrostatic [edit]
Hydrostatic gauges (such as the mercury cavalcade manometer) compare pressure to the hydrostatic strength per unit surface area at the base of a column of fluid. Hydrostatic estimate measurements are contained of the type of gas being measured, and can be designed to have a very linear calibration. They have poor dynamic response.
Piston [edit]
Piston-type gauges counterbalance the pressure of a fluid with a spring (for example tire-pressure gauges of comparatively low accurateness) or a solid weight, in which instance it is known every bit a deadweight tester and may be used for calibration of other gauges.
Liquid column (manometer) [edit]

The deviation in fluid acme in a liquid-column manometer is proportional to the pressure divergence:

Liquid-column gauges consist of a column of liquid in a tube whose ends are exposed to different pressures. The column will rise or autumn until its weight (a strength applied due to gravity) is in equilibrium with the pressure differential betwixt the 2 ends of the tube (a forcefulness applied due to fluid pressure). A very simple version is a U-shaped tube half-full of liquid, i side of which is connected to the region of involvement while the reference pressure (which might be the atmospheric pressure or a vacuum) is practical to the other. The difference in liquid levels represents the applied pressure. The pressure level exerted by a column of fluid of superlative h and density ρ is given by the hydrostatic pressure equation, P = hgρ. Therefore, the pressure difference between the applied pressure Pa and the reference pressure level P 0 in a U-tube manometer can be institute past solving Pa − P 0 = hgρ . In other words, the pressure on either end of the liquid (shown in bluish in the figure) must be balanced (since the liquid is static), and and so Pa = P 0 + hgρ .
In near liquid-column measurements, the outcome of the measurement is the top h, expressed typically in mm, cm, or inches. The h is also known every bit the pressure caput. When expressed as a pressure level head, pressure is specified in units of length and the measurement fluid must be specified. When accurateness is critical, the temperature of the measurement fluid must as well exist specified, because liquid density is a function of temperature. So, for instance, pressure head might be written "742.two mmHg" or "4.ii inH2O at 59 °F" for measurements taken with mercury or water as the manometric fluid respectively. The discussion "gauge" or "vacuum" may be added to such a measurement to distinguish between a pressure in a higher place or below the atmospheric pressure. Both mm of mercury and inches of h2o are common pressure level heads, which can be converted to S.I. units of pressure using unit of measurement conversion and the above formulas.
If the fluid existence measured is significantly dense, hydrostatic corrections may take to be made for the height between the moving surface of the manometer working fluid and the location where the pressure measurement is desired, except when measuring differential pressure of a fluid (for example, beyond an orifice plate or venturi), in which case the density ρ should be corrected past subtracting the density of the fluid being measured.[seven]
Although any fluid tin can be used, mercury is preferred for its high density (xiii.534 m/cmiii) and low vapour pressure. Its convex meniscus is advantageous since this means there volition be no pressure errors from wetting the glass, though under uncommonly clean circumstances, the mercury will stick to glass and the barometer may become stuck (the mercury can sustain a negative absolute pressure) even nether a strong vacuum.[8] For low pressure differences, light oil or water are commonly used (the latter giving rise to units of measurement such as inches water gauge and millimetres HiiO). Liquid-column pressure gauges have a highly linear scale. They have poor dynamic response because the fluid in the column may react slowly to a pressure change.
When measuring vacuum, the working liquid may evaporate and contaminate the vacuum if its vapor pressure is too high. When measuring liquid force per unit area, a loop filled with gas or a light fluid tin isolate the liquids to prevent them from mixing, but this can be unnecessary, for example, when mercury is used as the manometer fluid to measure differential pressure of a fluid such every bit water. Simple hydrostatic gauges can measure pressures ranging from a few torrs (a few 100 Pa) to a few atmospheres (approximately 1000 000 Pa).
A single-limb liquid-cavalcade manometer has a larger reservoir instead of i side of the U-tube and has a scale beside the narrower column. The cavalcade may be inclined to further amplify the liquid movement. Based on the use and structure, post-obit types of manometers are used[9]
- Elementary manometer
- Micromanometer
- Differential manometer
- Inverted differential manometer
McLeod guess [edit]

A McLeod gauge, drained of mercury
A McLeod gauge isolates a sample of gas and compresses it in a modified mercury manometer until the pressure is a few millimetres of mercury. The technique is very slow and unsuited to continual monitoring, simply is capable of good accuracy. Unlike other manometer gauges, the McLeod gauge reading is dependent on the composition of the gas, since the interpretation relies on the sample compressing equally an platonic gas. Due to the pinch procedure, the McLeod approximate completely ignores partial pressures from non-platonic vapors that condense, such every bit pump oils, mercury, and even water if compressed enough.
- Useful range: from around ten−four Torr[10] (roughly ten−2 Pa) to vacuums as high every bit ten−6 Torr (0.1 mPa),
0.1 mPa is the lowest directly measurement of pressure level that is possible with electric current technology. Other vacuum gauges tin can measure lower pressures, just only indirectly by measurement of other force per unit area-dependent properties. These indirect measurements must be calibrated to SI units by a direct measurement, most normally a McLeod gauge.[xi]
Aneroid [edit]
Aneroid gauges are based on a metallic pressure-sensing chemical element that flexes elastically under the consequence of a pressure difference across the element. "Aneroid" means "without fluid", and the term originally distinguished these gauges from the hydrostatic gauges described above. However, aneroid gauges tin be used to measure the pressure of a liquid as well equally a gas, and they are not the simply type of gauge that tin can operate without fluid. For this reason, they are often called mechanical gauges in modernistic language. Aneroid gauges are not dependent on the type of gas being measured, unlike thermal and ionization gauges, and are less probable to contaminate the system than hydrostatic gauges. The pressure sensing element may be a Bourdon tube, a diaphragm, a capsule, or a ready of bellows, which volition modify shape in response to the pressure of the region in question. The deflection of the pressure sensing element may be read past a linkage continued to a needle, or it may be read by a secondary transducer. The about common secondary transducers in modernistic vacuum gauges measure a modify in capacitance due to the mechanical deflection. Gauges that rely on a change in capacitance are often referred to as capacitance manometers.
Bourdon tube [edit]

The Bourdon pressure gauge uses the principle that a flattened tube tends to straighten or regain its circular form in cross-department when pressurized. (A party horn illustrates this principle.) This change in cross-section may exist hardly noticeable, involving moderate stresses within the rubberband range of easily workable materials. The strain of the textile of the tube is magnified by forming the tube into a C shape or even a helix, such that the entire tube tends to straighten out or uncoil elastically equally it is pressurized. Eugène Bourdon patented his gauge in France in 1849, and it was widely adopted because of its superior simplicity, linearity, and accuracy; Bourdon is now part of the Baumer group and nevertheless manufacture Bourdon tube gauges in France. Edward Ashcroft purchased Bourdon'southward American patent rights in 1852 and became a major manufacturer of gauges. Also in 1849, Bernard Schaeffer in Magdeburg, Federal republic of germany patented a successful diaphragm (see below) force per unit area guess, which, together with the Bourdon guess, revolutionized pressure measurement in manufacture.[12] Only in 1875 after Bourdon's patents expired, his visitor Schaeffer and Budenberg also manufactured Bourdon tube gauges.

An original 19th century Eugene Bourdon compound gauge, reading pressure both below and above atmospheric with great sensitivity
In practice, a flattened thin-wall, closed-end tube is connected at the hollow terminate to a fixed pipage containing the fluid pressure to be measured. Equally the pressure increases, the airtight finish moves in an arc, and this motion is converted into the rotation of a (segment of a) gear past a connecting link that is normally adjustable. A small-bore pinion gear is on the pointer shaft, then the motion is magnified further past the gear ratio. The positioning of the indicator menu behind the pointer, the initial arrow shaft position, the linkage length and initial position, all provide means to calibrate the pointer to indicate the desired range of pressure for variations in the behavior of the Bourdon tube itself. Differential force per unit area tin can be measured by gauges containing two different Bourdon tubes, with connecting linkages (but is more than normally measured via diaphragms or bellows and a balance organisation).
Bourdon tubes measures gauge pressure, relative to ambient atmospheric pressure, as opposed to accented pressure; vacuum is sensed as a opposite motion. Some aneroid barometers use Bourdon tubes closed at both ends (simply most use diaphragms or capsules, come across below). When the measured pressure is rapidly pulsing, such every bit when the gauge is near a reciprocating pump, an orifice brake in the connecting piping is ofttimes used to avoid unnecessary wear on the gears and provide an boilerplate reading; when the whole gauge is field of study to mechanical vibration, the instance (including the pointer and dial) tin be filled with an oil or glycerin. Typical high-quality modern gauges provide an accuracy of ±1% of span (Nominal diameter 100mm, Course 1 EN837-1), and a special loftier-accuracy gauge can be as accurate as 0.1% of full scale.[thirteen]
Force-balanced fused quartz Bourdon tube sensors work on the aforementioned principle simply uses the reflection of a axle of calorie-free from a mirror to sense the athwart displacement and current is applied to electromagnets to balance the force of the tube and bring the angular displacement back to zero, the current that is applied to the coils is used as the measurement. Due to the extremely stable and repeatable mechanical and thermal properties of quartz and the forcefulness balancing which eliminates nearly all physical movement these sensors can exist authentic to around 1 PPM of full scale.[14] Due to the extremely fine fused quartz structures which must be made by hand these sensors are generally limited to scientific and calibration purposes.
In the following illustrations of a compound judge (vacuum and gauge pressure), the case and window has been removed to show just the dial, pointer and process connexion. This particular gauge is a combination vacuum and pressure guess used for automotive diagnosis:

Indicator front with arrow and dial

Mechanical side with Bourdon tube
- The left side of the face, used for measuring vacuum, is calibrated in inches of mercury on its outer scale and centimetres of mercury on its inner calibration
- The right portion of the confront is used to measure fuel pump force per unit area or turbo boost and is scaled in pounds per square inch on its outer calibration and kg/cm2 on its inner scale.
Mechanical details [edit]

Stationary parts:
- A: Receiver block. This joins the inlet pipe to the fixed end of the Bourdon tube (1) and secures the chassis plate (B). The two holes receive screws that secure the case.
- B: Chassis plate. The dial is attached to this. It contains bearing holes for the axles.
- C: Secondary chassis plate. It supports the outer ends of the axles.
- D: Posts to join and space the two chassis plates.
Moving parts:
- Stationary finish of Bourdon tube. This communicates with the inlet pipe through the receiver cake.
- Moving end of Bourdon tube. This terminate is sealed.
- Pivot and pin pivot
- Link joining pivot pin to lever (5) with pins to allow articulation rotation
- Lever, an extension of the sector gear (7)
- Sector gear axle pivot
- Sector gear
- Indicator needle beam. This has a spur gear that engages the sector gear (7) and extends through the face to bulldoze the indicator needle. Due to the short distance between the lever arm link dominate and the pivot pin and the departure between the constructive radius of the sector gear and that of the spur gear, whatsoever motion of the Bourdon tube is greatly amplified. A small motion of the tube results in a large motion of the indicator needle.
- Hair spring to preload the gear train to eliminate gear lash and hysteresis
Diaphragm [edit]
A 2nd type of aneroid judge uses deflection of a flexible membrane that separates regions of different pressure level. The amount of deflection is repeatable for known pressures so the pressure level can be adamant by using calibration. The deformation of a thin diaphragm is dependent on the difference in pressure between its 2 faces. The reference face tin be open to atmosphere to measure guess force per unit area, open to a second port to measure out differential pressure, or tin can be sealed against a vacuum or other stock-still reference pressure to mensurate absolute pressure level. The deformation can be measured using mechanical, optical or capacitive techniques. Ceramic and metal diaphragms are used.
- Useful range: in a higher place x−2 Torr[15] (roughly ane Pa)
For accented measurements, welded pressure capsules with diaphragms on either side are often used.
shape:
- Flat
- Corrugated
- Flattened tube
- Sheathing
Bellows [edit]

A pile of pressure capsules with corrugated diaphragms in an aneroid barograph
In gauges intended to sense modest pressures or pressure differences, or require that an absolute pressure level be measured, the gear train and needle may be driven by an enclosed and sealed bellows chamber, called an aneroid. (Early barometers used a column of liquid such as water or the liquid metal mercury suspended by a vacuum.) This bellows configuration is used in aneroid barometers (barometers with an indicating needle and dial card), altimeters, altitude recording barographs, and the altitude telemetry instruments used in weather airship radiosondes. These devices use the sealed chamber equally a reference pressure and are driven by the external pressure. Other sensitive aircraft instruments such every bit air speed indicators and charge per unit of climb indicators (variometers) have connections both to the internal function of the aneroid bedroom and to an external enclosing sleeping accommodation.
Magnetic coupling [edit]
These gauges utilize the attraction of two magnets to translate differential pressure into movement of a dial pointer. Every bit differential pressure level increases, a magnet attached to either a piston or rubber diaphragm moves. A rotary magnet that is fastened to a arrow then moves in unison. To create unlike force per unit area ranges, the jump rate tin be increased or decreased.
Spinning-rotor guess [edit]
The spinning-rotor gauge works by measuring how a rotating brawl is slowed by the viscosity of the gas being measured. The ball is fabricated of steel and is magnetically levitated inside a steel tube closed at one end and exposed to the gas to exist measured at the other. The ball is brought upwardly to speed (nigh 2500 or 3800 rad/southward), and the deceleration rate is measured after switching off the drive, by electromagnetic transducers.[16] The range of the musical instrument is 5−v to 102 Pa (103 Pa with less accuracy). It is accurate and stable enough to exist used every bit a secondary standard. During the last years this type of gauge became much more user friendly and easier to operate. In the past the instrument was famous to requires some skill and knowledge to use correctly. For high accuracy measurements various corrections must exist practical and the ball must be spun at a pressure well below the intended measurement pressure for five hours earlier using. It is nearly useful in calibration and inquiry laboratories where loftier accuracy is required and qualified technicians are available.[17] Insulation vacuum monitoring of cryogenic liquids is a perfect suited awarding for this system too. With the inexpensive and long term stable, weldable sensor, that can exist separated from the more costly electronics/read it is a perfect fit to all static vacuums.
Electronic pressure instruments [edit]
- Metal strain gauge
- The strain gauge is generally glued (foil strain gauge) or deposited (thin-film strain judge) onto a membrane. Membrane deflection due to pressure causes a resistance change in the strain approximate which tin can be electronically measured.
- Piezoresistive strain gauge
- Uses the piezoresistive effect of bonded or formed strain gauges to notice strain due to applied pressure.
- Piezoresistive silicon pressure sensor
- The sensor is generally a temperature compensated, piezoresistive silicon force per unit area sensor called for its excellent performance and long-term stability. Integral temperature bounty is provided over a range of 0–50°C using laser-trimmed resistors. An additional laser-trimmed resistor is included to normalize pressure level sensitivity variations past programming the gain of an external differential amplifier. This provides good sensitivity and long-term stability. The two ports of the sensor, utilize pressure to the aforementioned unmarried transducer, please come across pressure level period diagram below.
![]()
This is an over-simplified diagram, but you can see the cardinal design of the internal ports in the sensor. The important item here to note is the "diaphragm" equally this is the sensor itself. Please annotation that is it slightly convex in shape (highly exaggerated in the drawing); this is important as it affects the accuracy of the sensor in use.
The shape of the sensor is important because information technology is calibrated to piece of work in the direction of air flow as shown by the Carmine arrows. This is normal operation for the pressure sensor, providing a positive reading on the display of the digital pressure meter. Applying pressure in the contrary direction can induce errors in the results equally the movement of the air pressure is trying to force the diaphragm to move in the contrary direction. The errors induced by this are small, but can be pregnant, and therefore it is always preferable to ensure that the more positive pressure is always applied to the positive (+ve) port and the lower pressure is practical to the negative (-ve) port, for normal 'guess pressure' application. The same applies to measuring the deviation between 2 vacuums, the larger vacuum should always be applied to the negative (-ve) port. The measurement of force per unit area via the Wheatstone Bridge looks something like this....

The constructive electrical model of the transducer, together with a basic signal conditioning circuit, is shown in the application schematic. The pressure sensor is a fully active Wheatstone bridge which has been temperature compensated and offset adjusted by means of thick motion-picture show, laser trimmed resistors. The excitation to the bridge is applied via a constant electric current. The low-level bridge output is at +O and -O, and the amplified span is set up by the gain programming resistor (r). The electrical design is microprocessor controlled, which allows for calibration, the additional functions for the user, such as Scale Selection, Data Concur, Nada and Filter functions, the Record function that stores/displays MAX/MIN.
- Capacitive
- Uses a diaphragm and pressure cavity to create a variable capacitor to detect strain due to applied pressure.
- Magnetic
- Measures the displacement of a diaphragm by means of changes in inductance (reluctance), LVDT, Hall effect, or by eddy current principle.
- Piezoelectric
- Uses the piezoelectric effect in certain materials such as quartz to measure the strain upon the sensing mechanism due to force per unit area.
- Optical
- Uses the physical change of an optical fiber to detect strain due to applied pressure level.
- Potentiometric
- Uses the motility of a wiper forth a resistive machinery to detect the strain caused by applied force per unit area.
- Resonant
- Uses the changes in resonant frequency in a sensing mechanism to measure out stress, or changes in gas density, caused by practical pressure.
Thermal electrical conductivity [edit]
By and large, every bit a real gas increases in density -which may indicate an increase in force per unit area- its ability to bear heat increases. In this type of estimate, a wire filament is heated past running current through it. A thermocouple or resistance thermometer (RTD) tin and so be used to measure the temperature of the filament. This temperature is dependent on the rate at which the filament loses heat to the surrounding gas, and therefore on the thermal conductivity. A mutual variant is the Pirani gauge, which uses a single platinum filament as both the heated element and RTD. These gauges are authentic from 10−3 Torr to ten Torr, simply their calibration is sensitive to the chemical composition of the gases existence measured.
Pirani (one wire) [edit]

Pirani vacuum estimate (open)
A Pirani judge consists of a metal wire open to the pressure existence measured. The wire is heated past a current flowing through it and cooled by the gas surrounding it. If the gas pressure level is reduced, the cooling effect volition decrease, hence the equilibrium temperature of the wire will increase. The resistance of the wire is a function of its temperature: by measuring the voltage across the wire and the current flowing through information technology, the resistance (and then the gas pressure level) tin can be determined. This type of gauge was invented by Marcello Pirani.
Two-wire [edit]
In two-wire gauges, one wire coil is used every bit a heater, and the other is used to measure temperature due to convection. Thermocouple gauges and thermistor gauges piece of work in this manner using a thermocouple or thermistor, respectively, to measure the temperature of the heated wire.
Ionization gauge [edit]
Ionization gauges are the well-nigh sensitive gauges for very low pressures (too referred to as hard or high vacuum). They sense pressure indirectly by measuring the electrical ions produced when the gas is bombarded with electrons. Fewer ions volition be produced by lower density gases. The calibration of an ion guess is unstable and dependent on the nature of the gases existence measured, which is non always known. They can be calibrated against a McLeod gauge which is much more stable and contained of gas chemistry.
Thermionic emission generates electrons, which collide with gas atoms and generate positive ions. The ions are attracted to a suitably biased electrode known equally the collector. The electric current in the collector is proportional to the charge per unit of ionization, which is a part of the pressure in the system. Hence, measuring the collector current gives the gas pressure level. There are several sub-types of ionization gauge.
- Useful range: x−10 - 10−3 torr (roughly 10−8 - 10−one Pa)
About ion gauges come up in two types: hot cathode and common cold cathode. In the hot cathode version, an electrically heated filament produces an electron beam. The electrons travel through the judge and ionize gas molecules around them. The resulting ions are nerveless at a negative electrode. The current depends on the number of ions, which depends on the pressure in the gauge. Hot cathode gauges are accurate from 10−3 Torr to 10−10 Torr. The principle behind cold cathode version is the same, except that electrons are produced in the discharge of a high voltage. Cold cathode gauges are accurate from ten−2 Torr to 10−9 Torr. Ionization approximate scale is very sensitive to construction geometry, chemical composition of gases being measured, corrosion and surface deposits. Their calibration can be invalidated by activation at atmospheric pressure or low vacuum. The limerick of gases at high vacuums will usually be unpredictable, so a mass spectrometer must be used in conjunction with the ionization estimate for authentic measurement.[xviii]
Hot cathode [edit]
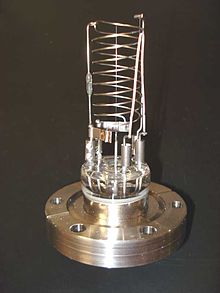
Bayard–Alpert hot-cathode ionization gauge
A hot-cathode ionization gauge is equanimous mainly of three electrodes acting together as a triode, wherein the cathode is the filament. The iii electrodes are a collector or plate, a filament, and a filigree. The collector current is measured in picoamperes by an electrometer. The filament voltage to basis is usually at a potential of xxx volts, while the grid voltage at 180–210 volts DC, unless at that place is an optional electron bombardment feature, past heating the grid, which may have a high potential of approximately 565 volts.
The most mutual ion estimate is the hot-cathode Bayard–Alpert judge, with a small ion collector within the grid. A glass envelope with an opening to the vacuum can environs the electrodes, only unremarkably the nude gauge is inserted in the vacuum bedchamber directly, the pins existence fed through a ceramic plate in the wall of the chamber. Hot-cathode gauges tin exist damaged or lose their calibration if they are exposed to atmospheric pressure or even low vacuum while hot. The measurements of a hot-cathode ionization gauge are ever logarithmic.
Electrons emitted from the filament move several times in back-and-forth movements around the grid before finally entering the grid. During these movements, some electrons collide with a gaseous molecule to course a pair of an ion and an electron (electron ionization). The number of these ions is proportional to the gaseous molecule density multiplied by the electron electric current emitted from the filament, and these ions pour into the collector to grade an ion current. Since the gaseous molecule density is proportional to the pressure, the force per unit area is estimated by measuring the ion current.
The depression-pressure sensitivity of hot-cathode gauges is limited past the photoelectric effect. Electrons hit the grid produce x-rays that produce photoelectric dissonance in the ion collector. This limits the range of older hot-cathode gauges to 10−8 Torr and the Bayard–Alpert to most 10−10 Torr. Additional wires at cathode potential in the line of sight betwixt the ion collector and the grid prevent this effect. In the extraction type the ions are not attracted by a wire, only past an open cone. Equally the ions cannot decide which part of the cone to striking, they laissez passer through the hole and course an ion beam. This ion beam can be passed on to a:
- Faraday cup
- Microchannel plate detector with Faraday cup
- Quadrupole mass analyzer with Faraday cup
- Quadrupole mass analyzer with microchannel plate detector and Faraday cup
- Ion lens and dispatch voltage and directed at a target to class a sputter gun. In this case a valve lets gas into the grid-cage.
Cold cathode [edit]

Penning vacuum gauge (cut-away)
In that location are two subtypes of cold-cathode ionization gauges: the Penning approximate (invented past Frans Michel Penning), and the inverted magnetron, besides called a Redhead gauge. The major difference between the two is the position of the anode with respect to the cathode. Neither has a filament, and each may require a DC potential of most 4 kV for operation. Inverted magnetrons can mensurate downwards to 1×10−12 Torr.
As well, cold-cathode gauges may be reluctant to commencement at very low pressures, in that the nigh-absenteeism of a gas makes it difficult to constitute an electrode electric current - in item in Penning gauges, which use an axially symmetric magnetic field to create path lengths for electrons that are of the order of metres. In ambient air, suitable ion-pairs are ubiquitously formed by cosmic radiations; in a Penning gauge, pattern features are used to ease the set-upwardly of a discharge path. For case, the electrode of a Penning judge is commonly finely tapered to facilitate the field emission of electrons.
Maintenance cycles of cold cathode gauges are, in general, measured in years, depending on the gas blazon and pressure level that they are operated in. Using a cold cathode gauge in gases with substantial organic components, such as pump oil fractions, can issue in the growth of delicate carbon films and shards within the gauge that eventually either short-circuit the electrodes of the gauge or impede the generation of a discharge path.
| Physical phenomena | Instrument | Governing equation | Limiting factors | Practical pressure range | Ideal accuracy | Response time |
|---|---|---|---|---|---|---|
| Mechanical | Liquid column manometer | atm. to ane mbar | ||||
| Mechanical | Capsule punch guess | Friction | grand to 1 mbar | ±five% of full scale | Slow | |
| Mechanical | Strain gauge | k to ane mbar | Fast | |||
| Mechanical | Capacitance manometer | Temperature fluctuations | atm to 10−half-dozen mbar | ±1% of reading | Slower when filter mounted | |
| Mechanical | McLeod | Boyle's constabulary | ten to ten−3 mbar | ±10% of reading between x−four and 5⋅10−two mbar | ||
| Transport | Spinning rotor (elevate) | ten−one to 10−7 mbar | ±2.5% of reading between 10−7 and 10−ii mbar 2.5 to thirteen.five% between ten−2 and 1 mbar | |||
| Ship | Pirani (Wheatstone bridge) | Thermal conductivity | 1000 to 10−3 mbar (const. temperature) 10 to x−3 mbar (const. voltage) | ±six% of reading between 10−ii and 10 mbar | Fast | |
| Ship | Thermocouple (Seebeck upshot) | Thermal conductivity | 5 to 10−3 mbar | ±x% of reading betwixt 10−2 and 1 mbar | ||
| Ionization | Common cold cathode (Penning) | Ionization yield | 10−2 to x−seven mbar | +100 to -l% of reading | ||
| Ionization | Hot cathode (ionization induced by thermionic emission) | Low electric current measurement; parasitic x-ray emission | x−iii to 10−10 mbar | ±10% betwixt 10−vii and x−4 mbar ±twenty% at 10−3 and 10−9 mbar ±100% at 10−10 mbar |
Dynamic transients [edit]
When fluid flows are not in equilibrium, local pressures may be higher or lower than the average pressure in a medium. These disturbances propagate from their source as longitudinal pressure variations along the path of propagation. This is too called sound. Sound pressure is the instantaneous local pressure divergence from the average pressure caused by a sound wave. Audio pressure can be measured using a microphone in air and a hydrophone in water. The effective sound pressure is the root mean square of the instantaneous sound pressure level over a given interval of time. Audio pressures are normally small and are often expressed in units of microbar.
- frequency response of pressure sensors
- resonance
Calibration and standards [edit]

Dead-weight tester. This uses known calibrated weights on a piston to generate a known pressure.
The American Society of Mechanical Engineers (ASME) has developed 2 separate and distinct standards on pressure level measurement, B40.100 and PTC nineteen.ii. B40.100 provides guidelines on Force per unit area Indicated Dial Type and Pressure Digital Indicating Gauges, Diaphragm Seals, Snubbers, and Pressure Limiter Valves. PTC 19.two provides instructions and guidance for the accurate conclusion of force per unit area values in back up of the ASME Functioning Examination Codes. The option of method, instruments, required calculations, and corrections to be practical depends on the purpose of the measurement, the allowable uncertainty, and the characteristics of the equipment existence tested.
The methods for pressure measurement and the protocols used for data transmission are also provided. Guidance is given for setting upward the instrumentation and determining the uncertainty of the measurement. Information regarding the instrument type, design, applicable pressure range, accuracy, output, and relative cost is provided. Information is likewise provided on pressure-measuring devices that are used in field environments i.e., piston gauges, manometers, and low-accented-force per unit area (vacuum) instruments.
These methods are designed to assist in the evaluation of measurement uncertainty based on electric current technology and engineering noesis, taking into account published instrumentation specifications and measurement and awarding techniques. This Supplement provides guidance in the use of methods to establish the pressure-measurement uncertainty.
European (CEN) Standard [edit]
- EN 472 : Pressure gauge - Vocabulary.
- EN 837-1 : Pressure gauges. Bourdon tube pressure gauges. Dimensions, metrology, requirements and testing.
- EN 837-two : Pressure gauges. Selection and installation recommendations for pressure gauges.
- EN 837-iii : Pressure gauges. Diaphragm and capsule pressure gauges. Dimensions, metrology, requirements, and testing.
Us ASME Standards [edit]
- B40.100-2013: Pressure gauges and Gauge attachments.
- PTC xix.2-2010 : The Performance test lawmaking for pressure measurement.
See also [edit]
- Air core guess
- Deadweight tester
- Force guess
- Judge
- Isoteniscope
- Piezometer
- Sphygmomanometer
- Time pressure guess
- Tire-pressure gauge
- Vacuum engineering science
References [edit]
- ^ NIST
- ^ a b Staff (2016). "2 - Diving physics". Guidance for Diving Supervisors (IMCA D 022 Baronial 2016, Rev. 1 ed.). London, Britain: International Marine Contractors' Association. p. 3.
- ^ Page 2-12.
- ^ "Understanding Vacuum Measurement Units". 9 February 2013.
- ^ Methods for the Measurement of Fluid Menstruum in Pipes, Role ane. Orifice Plates, Nozzles and Venturi Tubes. British Standards Found. 1964. p. 36.
- ^ Transmission of Barometry (WBAN) (PDF). U.S. Government Printing Office. 1963. pp. A295–A299.
- ^ [Was: "fluidengineering.co.nr/Manometer.htm". At 1/2010 that took me to bad link. Types of fluid Manometers]
- ^ "Techniques of High Vacuum". Tel Aviv University. 2006-05-04. Archived from the original on 2006-05-04.
- ^ Beckwith, Thomas Yard.; Marangoni, Roy D. & Lienhard 5, John H. (1993). "Measurement of Low Pressures". Mechanical Measurements (Fifth ed.). Reading, MA: Addison-Wesley. pp. 591–595. ISBN0-201-56947-7.
- ^ The Engine Indicator Canadian Museum of Making
- ^ Boyes, Walt (2008). Instrumentation Reference Volume (Quaternary ed.). Butterworth-Heinemann. p. 1312.
- ^ "Characterization of quartz Bourdon-type high-pressure transducers". ResearchGate . Retrieved 2019-05-05 .
- ^ Production brochure from Schoonover, Inc
- ^ A. Chambers, Basic Vacuum Technology, pp. 100–102, CRC Press, 1998. ISBN 0585254915.
- ^ John F. O'Hanlon, A User's Guide to Vacuum Engineering science, pp. 92–94, John Wiley & Sons, 2005. ISBN 0471467154.
- ^ Robert M. Besançon, ed. (1990). "Vacuum Techniques". The Encyclopedia of Physics (3rd ed.). Van Nostrand Reinhold, New York. pp. 1278–1284. ISBN0-442-00522-9.
- ^ Nigel S. Harris (1989). Mod Vacuum Practice. McGraw-Hill. ISBN978-0-07-707099-1.
Sources [edit]
- US Navy (one Dec 2016). U.Southward. Navy Diving Manual Revision seven SS521-AG-PRO-010 0910-LP-115-1921 (PDF). Washington, DC.: US Naval Sea Systems Command. Archived (PDF) from the original on December 28, 2016.
External links [edit]
![]()
- Home Made Manometer
- Manometer
What Tool Measures Air Pressure,
Source: https://en.wikipedia.org/wiki/Pressure_measurement
Posted by: handyowly1985.blogspot.com




0 Response to "What Tool Measures Air Pressure"
Post a Comment